It’s hard to miss calcium. Most trusted health resources, along with the ever-present media, extol the virtues of calcium as if it were the only mineral our bodies require for good health. We can thank the American Dairy Council for milking calcium and putting it in the spotlight.
But calcium can’t act alone. It needs magnesium. These two minerals are so critical to many functions in the body that not having enough magnesium—and an excess of calcium—can cause serious health complications.
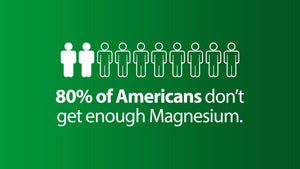
Find out why so many of us are magnesium deficient.
Muscle Health
Consider any movement by the body, for example. Electrical impulses transmit signals to the nerves and brain, and movement occurs. But the conductor for these impulses is calcium which enters the cells through calcium channels operated by magnesium. Once calcium does its work, magnesium helps the body get rid of the calcium before it crystallizes.
If there’s no magnesium to help the body eliminate calcium, then the calcium builds up in the cells. That’s why magnesium is often called nature’s calcium channel blocker.[1,2,3]
Calcium works in the muscles, too, contracting muscle fibers while magnesium relaxes them. Too much calcium and your muscles will begin to twitch or spasm.
Bone Health
We already know that calcium protects and keeps bones and teeth healthy. But without magnesium, the body cannot:
- Adequately absorb calcium.*
- Stimulate calcitonin, a hormone that draws calcium from the blood and tissues back into the bones.*
- Suppress parathyroid, another hormone that breaks down bone.*
- Convert vitamin D into its active form for calcium absorption.*
- Activate an enzyme required for new bone to form.*
- Regulate calcium transport.*
Clearly, even a mild deficiency in magnesium can radically affect bone health.
To ensure you get the nutrients you need, consider supplementing.
Calcium & Magnesium
Clearly, focusing on a single nutrient like calcium, to the exclusion of all of the other vitally important nutrients like magnesium, can lead to excess calcium and depleted magnesium—and an invitation for more troubling and serious conditions later on.
So to stay healthy, make sure you take calcium AND magnesium. And stay away from supplements that contain both—they’re usually less absorbed forms that are often quickly excreted before they can do any good.
Sources:
Dean C, MD, ND. The Magnesium Miracle, Ballantine Books: New York, 2007.
Cited Sources:
- Iseri LT & French JH. “Magnesium: nature’s physiologic calcium blocker.” Am Heart J, 108, 188-193, 1984.
- Seelig MS. “Cardiovascular reactions to stress intensified by magnesium deficit in consequences of magnesium deficiency on the enhancement of stress reactions; preventive and therapeutic implications: a review,” J Am Coll Nutr, 13, 5:429-446, 1994.
- Rodale JR. Magnesium: The Nutrient that Could Change Your Life, Rodale Press: Emmaus, PA, 1971.
- Thomas AJ, et al. “Ca, Mg, and P status of elderly inpatients: dietary intake, metabolic balance studies and biochemical status,” Br J Nutr, 62, 211-219, 1989.
- Bunker VW. “Osteoporosis in the elderly,” Br J Biomed Sci, 51, 3:228-240, 1994.
- Brodowski J. “Levels of ionized magnesium in women with various stages of postmenopausal osteoporosis progression evaluated on the basis of densitometric examinations,” Przegl Lek, 57, 12:714-716, 2000.
- Sojka JE & Weaver CM. “Magnesium supplementation and osteoporosis,” Nutrition Reviews, 53, 71, 1995.
- Hall WD, et al. “Risk factors for kidney stones in older women in the southern United States,” Am J Med Sci, 322, 1:12-18, 2001.
- Bunce GE, et al. “Distribution of calcium and magnesium in rat kidney homogenate fractions accompanying magnesium deficiency induced nephrocalcinosis,” Exp Mol Pathol, 21, 1:16-28, 1974.
- Johannson G, et al. “Effects of magnesium hydroxide in renal stone disease,” J Am Coll Nutr, 1, 2, 1982.
- Prien EL. “Magnesium oxide-pyridoxine therapy for recurring calcium oxalate urinary calculi,” J Urology, 112, 509-551, 1974.
- Johannson G, et al. “Biochemical and clinical effects of prophylactic treatment of renal calcium stones with magnesium hydroxide,” J Urology, 124, 770-774, 1980.